CHEMOS Clinical Trial - CONCLUDED
Prof. Riccardo Torta
Aree / Gruppi di ricerca
Partecipanti al progetto
- Brusa Paola (Coordinatore/trice)
- Baratta Francesca (Borsista)
- Milla Paola (Docente)
- Prof. Riccardo Torta (Docente)
Descrizione del progetto
Members of other units:
Dr. N. Birocco
Dr. F. Cattel
Prof. L. Cattel
Dr. L. Ciuffreda
Dr. M. Schena
S. Storto
Partners:
Medical Oncology 1, San Giovanni Battista Hospital, Turin, Italy
Hospital Pharmacy, San Giovanni Battista Hospital, Turin, Italy
S.C.D.U. - Psicologia Clinica e Oncologica, Dept. di Neuroscienze e Oncologia, University of Turin, Italy
Sponsors:
Dr. L. Ciuffreda, Medical Oncology 1, San Giovanni Battista Hospital, Turin, Italy
Description:
This study, which full title is “Approccio al counseling dei pazienti oncologici sottoposti a terapia orale: apporto del gruppo multidisciplinare medico-infermiere-farmacista-psiconcologo”, started in 2010. It’s an observational randomized open clinical trial.
Recently many oncologic medicinal products for oral administration have been introduced. Oral administration shows many advantages respect to the iv one, as improvement of life style, reduction of venous infections and reduction of health spending, concerning above all medical devices and health assistance. For the medical doctor the problem, on the contrary, is the poor check on the adherence to the therapy by his patients.
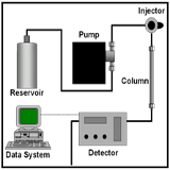
The aim of this study is the evaluation of the efficacy of the counseling managed with a multitask group formed by medical doctor-nurse-pharmacist-pshyconcologist: it is studied the improvement of adherence to the oral therapy of oncologic patients respect to those which received the counseling from medical doctor-nurse only. Erlotinib, Sorafenib o Sunitinib have been chosen as API to select the patients to enroll. Some initial questionnaires have been administrated to the enrolled subjects at the beginning of the trial, moreover, during the routinely hospital check previewed by therapeutic protocol, the evaluation of steady state plasmatic level of the administered API has been ascertain by HPLC analysis.
At the end of 2014, all the collected data are analyzed in order to obtain the conclusions of the study.
Keywords:
Chemos, HPLC, psycho-oncology, oncological patients, anticancer drugs, clinical trial.




