Synaptic plasticity and ion channels remodelling in hippocampus during stress (PRIN-MIUR) - CONCLUDED
Aree / Gruppi di ricerca
Partecipanti al progetto
- Carbone Emilio (Coordinatore/trice)
- (Assegnista)
- Calorio Chiara (Dottorando/a)
- Franchino Claudio (Tecnico/a)
- (Assegnista)
Descrizione del progetto
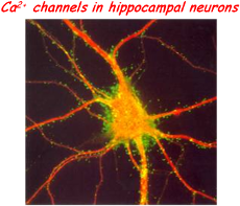
Members of other units:
Fabio Benfenati (Genova; coordinatore nazionale)
Egidio D’Angelo (Pavia)
Cherubini Enrico (Trieste)
Conti Fiorenzo (Ancona)
Valtorta Flavia (Milano)
Matteoli Michela (Milano)
Diaspro Alberto (Genova)
Daniela Pietrobon (Padova)
Alberto Pasquarelli (Ulm)
Partners:
University of Pavia
University of Padova
University of Milano
University of Genova
University of Marche
IIT (Genova)
SISSA (Trieste)
University “Vita Salute San Raffaele” (Milano)
Institute for Electronic Devices and Circuits, University of Ulm (Germany)
Sponsors:
Ministero dell’Istruzione, Università e Ricerca (MIUR)
Fondi: PRIN 2012/2015
Description
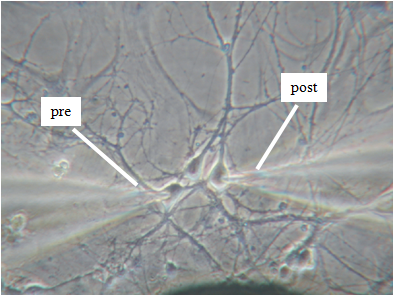
As for most neurological and psychiatric disorders, a synapticdysfunction is at the basis of chronic stress-induced neuropathies. Common cause of these synaptopathies is the evident unbalancing of excitatory/inhibitory (E/I) synapses occurring progressively with time in limbic brain structures: hippocampus, amygdala and prefrontal cortex. This is most evident during “prenatal stress”, which among other effects, is known to elevate basal corticosterone (CC) levels and to decrease glucocorticoid receptors expression in the hippocampus and prefrontal cortex. The involvement of hippocampal memory circuits in prenatal stress is supported by recent findings showing that pups of stressed pregnant mice are often accompanied by a reduced capacity of spatial learning and memory tests which are attributed to a reduced NMDA-mediated long-term potentiation (LTP) in CA1 area of hippocampus. Alterations of LTP and memory functions induced by prenatal stress are likely to derive from the modified equilibrium of excitatory and inhibitory GABAergic synapses which is particularly relevant in developing hippocampal neurons. Concerning this issue, there are few controversial findings; in particular about the changes of expression densities and gating properties of ion channels and GABAergic/glutamatergic receptors responsible of the E/I balancing controlling the switch from asynchronous to synchronous neuronal firings during development.
The main objectives of the project are: 1) understanding the role of post-synaptic L-type Ca2+ channels (Cav1.2 and Cav1.3) and Ca2+-dependent K+ channels (SK and BK) responsible for the burst firing behavior of developing hippocampal networks of normal and maternally stressed animals and, 2) identify the role that pre-synaptic Ca2+channels and synapsins I/II play in the GABAergic/glutamatergic plasticity changes of hippocampal networks induced by chronic maternal stress or chronic exposure to “stressors” (cortisol and corticosterone).
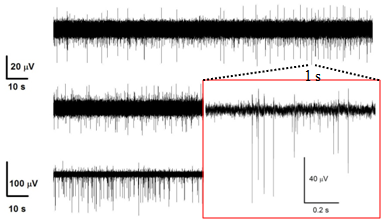
For the project will be used hippocampal primary cell cultures from embryos at the 18th day of gestation from normal and chronically stressed mother mice. We will analyze their burst firing behavior, pre- and post-synaptic ion currents and the elementary properties of GABAergic and glutamatergic receptors with time in culture by using KO mice lacking synapsins I/II or Cav1.3 channels and most advanced electrophysiological techniques: traditional and diamond-based MEAs, current-clamp and voltage-clamp recordings. In this way, we expect to have a complete view of the altered activity of chronically stressed hippocampus by monitoring the entire neuronal network activity (MEA recordings), the excitability of single neurons (current-clamp recordings), the somatic ion currents and the pre/post synaptic activity in identified GABAergic and glutamatergic synapses (voltage-clamp recordings) during development and stress disorder.
Concerning the two objectives we aim at: 1) establishing the changes of expression density and functional gating properties that SK, BK and L-type Ca2+ channels undergo in embryos after maternal stress disorders and 2) identifying the role that presynaptic Ca2+ channels (Cav2.1, Cav2.2 and Cav2.3) and synapsin I/II play in the regulation of neurotransmission and E/I plasticity changes induced by chronic stress.
References:
- Baldelli P et al. 2005. BDNF enhances GABA release probability and non-uniform distribution of N- and P/Q-type channels on release sites of hippocampal inhibitory synapses. Journal of Neuroscience 25: 3358-3368
- Marcantoni A et al. 2010. Loss of Cav1.3 channels reveals the critical role of L- and BK-channel coupling in pace-making mouse adrenal chromaffin cells. Journal of Neuroscience 30: 491-504
- Vandael DHF et al. 2012. Cav1.3-driven SK channel activation regulates pacemaking and spike frequency adaptation in mouse chromaffin cells. Journal of Neuroscience 32: 16345-16359
Keywords:
Chronic prenatal stress, Glucocorticoid receptors, Corticosteroids, Presynaptic calcium channels, Postsynaptic sodium, Calcium and potassium channels, GABAergic and glutamatergic receptors, Excitatory and inhibitory synapses; Hippocampal networks activity, LTP and neuronal plasticity, Current-clamp, Voltage-clamp and MEA recordings, Inhibitory post-synaptic currents (IPSCs), Excitatory post-synaptic currents (EPSCs), Action potentials
Links:
Graduatorie finali MIUR-area5 (allegato presente anche alla voce Documentazione)




