Innovative Pharmaceutical and Cosmetic Technology and Nanotechnology Group (i-PHARCOTEC)
Componenti
- Cavalli Roberta (Coordinatore/Coordinatrice)
- Argenziano Monica (Ricercatore/Ricercatrice)
- Bastiancich Chiara (Ricercatore/Ricercatrice)
- Battaglia Luigi Sebastiano (Docente)
- Chirio Daniela (Docente)
- Gallarate Marina (Docente)
- Peira Elena (Docente)
- Sapino Simona (Tecnico/a)
- Scomparin Anna (Docente)
- Spagnolo Rita (Tecnico/a)
- Ugazio Elena (Docente)
- Ansari Irfan Aamer (Dottorando/a)
- Bozza Annalisa (Dottorando/a)
- Chindamo Giulia (Dottorando/a)
- Molinar Chiara (Dottorando/a)
Contatti

Settore ERC
Attività
Il gruppo i-PHARCOTEC possiede una consolidata esperienza nella progettazione, preparazione e caratterizzazione di formulazioni farmaceutiche innovative costituite da nano e/o microparticelle a matrice polimerica, lipidica e inorganica. In particolare, vengono sviluppate nanoparticelle polimeriche, nanoparticelle solide lipidiche, liposomi, coniugati polimero-farmaco, nanoparticelle a base di ciclodestrine, nanocristalli, sistemi a base di nanovescicole (nanobolle, nanocapsule), nano e microemulsioni per applicazioni farmaceutiche e/o cosmetiche. Inoltre, parte della ricerca è focalizzata allo sviluppo di sistemi direzionati, sfruttando strategie su più fronti.
Tali approcci formulativi sono tutti guidati dal principio attivo:
- vengono sviluppate strategie formulative in funzione delle caratteristiche chimico-fisiche del farmaco/sostanza funzionale da caricare all'interno dei micro/nanosistemi, tra cui idro/lipofilia, peso molecolare, stabilità chimica e termica;
- le dimensioni e le proprietà superficiali dei micro/nanosistemi sono ottimizzate al fine di raggiungere il sito bersaglio
- la struttura composita del micro/nanosistema è progettata per migliorare la solubilità del farmaco e/o per ottenere il suo rilascio prolungato
|
Le attività di ricerca in campo farmaceutico sono focalizzate principalmente sui seguenti argomenti:
Attività di ricerca in corso in campo cosmetico:
|
|
|
Principali tecnologie disponibili:
|
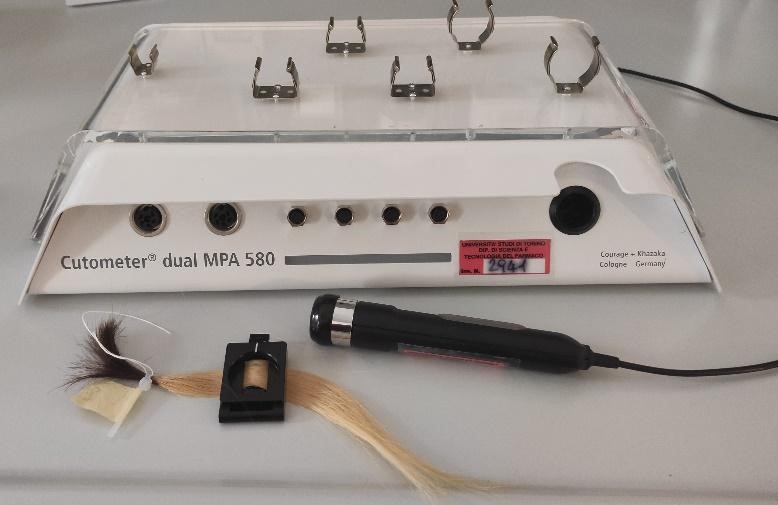 Cutometro Cutometro |
- Azienda Ospedaliero-Universitaria Città della Salute e della Scienza di Torino, Torino
- S.C. Farmacia Ospedaliera A.O. Ordine Mauriziano, Torino
- Istituto di Candiolo - IRCCS, Candiolo
- Società Prodotti Antibiotici S.p.A
- Dipartimento di Scienza della Salute, Università del Piemonte Orientale, Novara
- Dipartimento di Scienze del Farmaco, Università del Piemonte Orientale, Novara
- Centro di Riferimento Oncologico (CRO) di Aviano IRCCS, Aviano
- Neuroengineering and Neuroprosthesis Unit at the Bioengineering Institute. University Miguel Hernández (Spain)
- Department of Pharmaceutical Sciences, School of Health Sciences of Polytechnic Institute of Guarda (Guarda, Portugal)
- Instituto de Investigaciones Fisicoquímicas Teóricas y Aplicadas (INIFTA) La Plata (Argentina)
- Department of Physiology and Pharmacology, Sackler Faculty of Medicine, Tel Aviv University, Tel Aviv (Israel), Prof. Ronit Satchi-Fainaro
- Research Institute for Medicines, iMed.ULisboa, Faculty of Pharmacy, University of Lisbon, Lisboa (Portugal), Prof. Helena Florindo
- Advanced Drug Delivery and Biomaterials, Louvain Drug Research Institute, UCLouvain (Belgium)
- Gliomagenesis and Microenvironment, Institute of NeuroPhysiopathology, Aix-Marseille University (France)
To develop innovative and smart nanosystems for drug delivery by exploiting drug-driven formulation approaches.
The i-PHARCOTEC group owns a widespread and established expertise in the design, preparation and characterization of innovative drug formulations by means of nano and/or microparticles, based on polymer, lipid and inorganic matrixes. In particular, polymeric nanoparticles, solid lipid nanoparticles, liposomes, polymer-drug conjugates, cyclodextrin-based nanoparticles, nanocrystals, nanovesicle-based systems (i.e. nanobubbles, nanocapsules), nano and microemulsion are developed for pharmaceutical and/or cosmetic applications. Moreover, such research has also been focused on the development of targeted nanosystems, by using multi-pronged strategies.
Such formulative approaches are all drug-driven;that is:
- formulation strategies are developed owing to the physico-chemical features of the drug/functional ingredient to be loaded within the micro/nanosystems, including hydro/lipophilicity, molecular weight, chemical and thermal stability;
- size and surface properties of micro/nanosystems are optimized, in order to enable them to deliver their cargo to its specific target site;
- micro/nanosystem composite structure is designed in order to enhance the drug solubility and/or to achieve its sustained release.
|
Research activities in pharmaceutical field are mainly focused on the following topics:
Research activities in progress in cosmetic field:
|
Solid lipid nanoparticles
In-situ gelling microemulsions
Ocular 3-compartments flow cell
|
|
Main available technologies:
|
 Cutometer Cutometer |
- University Hospital Città della Salute e della Scienza di Torino, Torino
- S.C. Farmacia Ospedaliera A.O. Ordine Mauriziano, Torino
- Candiolo Cancer Institute, FPO—IRCCS, Candiolo
- Società Prodotti Antibiotici S.p.A
- Department of Health Sciences, Università del Piemonte Orientale, Novara
- Department of Pharmaceutical Sciences, Università del Piemonte Orientale, Novara
- Centro di Riferimento Oncologico (CRO) di Aviano IRCCS, Aviano
- Neuroengineering and Neuroprosthesis Unit at the Bioengineering Institute. University Miguel Hernández (Spain)
- Department of Pharmaceutical Sciences, School of Health Sciences of Polytechnic Institute of Guarda (Guarda, Portugal)
- Instituto de Investigaciones Fisicoquímicas Teóricas y Aplicadas (INIFTA) La Plata (Argentina)
- Department of Physiology and Pharmacology, Sackler Faculty of Medicine, Tel Aviv University, Tel Aviv (Israel), Prof. Ronit Satchi-Fainaro
- Research Institute for Medicines, iMed.ULisboa, Faculty of Pharmacy, University of Lisbon, Lisboa (Portugal), Prof. Helena Florindo
- Advanced Drug Delivery and Biomaterials, Louvain Drug Research Institute, UCLouvain (Belgium)
- Gliomagenesis and Microenvironment, Institute of NeuroPhysiopathology, Aix-Marseille University (France)
Progetti
- NODES - Nord Ovest Digitale E Sostenibile
- An Innovative Nanotechnology for Glioma Chemotherapy
- NanoLipid@NasalDevice: Nanocarriers lipidici nasali come device per la prevenzione e il trattamento di patologie cerebrovascolari
- SERENDIPITi - Sviluppo di sensori per la determinazione delle diverse forme di ferro per la diagnosi precoce di patologie neurodegenerative
- Sviluppo di nanomedicine per l’immunoterapia e la chemioterapia per il trattamento del glioblastoma
- Sviluppo di sensori elettrochimici per la determinazione delle diverse forme di ferro per la diagnosi precoce di patologie neurodegenerative
- Development of oxidic and polymeric materials for stimuli responsive applications (OXYPOLISTI) - CONCLUDED
- Advanced nanosystems for ophthalmic administration
- Applying nanotechnology for brain tumor immunotherapeutics
- Asymmetrical flow field-flow fractionation (AF4) technique in pharmaceutical and biomedical applications.
- Effect of nano-TiO2 on transdermal permeation through pig skin as a function of characteristics of the drug selected
- Encapsulation of antitumoral drugs and curcumin as Pgp inhibitor in solid lipid nanoparticles (SLN)
- Progetto Actavis Italy Spa (Pro-ACT)
- Progetto Mastelli srl (MAST)
- Preclinical study targeting mechanosensitive Ca2+ channels for Cerebral Cavernous Malformations therapy and early diagnosis - MECACCM
Prodotti della ricerca
Wang M, Malfanti A, Bastiancich C, Preat V. (2023) Synergistic effect of doxorubicin lauroyl hydrazone derivative delivered by alpha-tocopherol succinate micelles for the treatment of glioblastoma. International journal of pharmaceutics: X 5 100147 [DOI PMID]
Argenziano M, Bessone F, Dianzani C, Cucci MA, Grattarola M, Pizzimenti S, Cavalli R. (2022) Ultrasound-Responsive Nrf2-Targeting siRNA-Loaded Nanobubbles for Enhancing the Treatment of Melanoma. Pharmaceutics 14(2) [DOI PMID]
Bozzato E, Tsakiris N, Paquot A, Muccioli GG, Bastiancich C, Preat V. (2022) Dual-drug loaded nanomedicine hydrogel as a therapeutic platform to target both residual glioblastoma and glioma stem cells. International journal of pharmaceutics 628 122341 [DOI PMID]
Naked and Decorated Nanoparticles Containing H2S-Releasing Doxorubicin: Preparation, Characterization and Assessment of Their Antitumoral Efficiency on Various Resistant Tumor Cells
2022-01-01 Elena PEIRA; Daniela Chirio; Simona Sapino; Konstantin CHEGAEV; Giulia Chindamo; IRIS CHIARA SALAROGLIO; Chiara Riganti; Marina GALLARATE https://iris.unito.it/handle/2318/1885286
Chirio D, Sapino S, Chindamo G, Peira E, Vercelli C, Riganti C, Manzoli M, Gambino G, Re G, Gallarate M. (2022) Doxorubicin-Loaded Lipid Nanoparticles Coated with Calcium Phosphate as a Potential Tool in Human and Canine Osteosarcoma Therapy. Pharmaceutics 14(7) [DOI PMID]
Chirio D, Peira E, Sapino S, Chindamo G, Oliaro-Bosso S, Adinolfi S, Dianzani C, Baratta F, Gallarate M. (2021) A New Bevacizumab Carrier for Intravitreal Administration: Focus on Stability. Pharmaceutics 13(4) [DOI PMID]
Giovannelli L, Milanesi A, Ugazio E, Fracchia L, Segale L. (2021) Effect of Methyl-beta-Cyclodextrin and Trehalose on the Freeze-Drying and Spray-Drying of Sericin for Cosmetic Purposes. Pharmaceuticals (Basel, Switzerland) 14(3) [DOI PMID]
Peira E, Chindamo G, Chirio D, Sapino S, Oliaro-Bosso S, Rebba E, Ivanchenko P, Gallarate M. (2021) Assessment of In-Situ Gelling Microemulsion Systems upon Temperature and Dilution Condition for Corneal Delivery of Bevacizumab. Pharmaceutics 13(2) [DOI PMID]
Scutera S, Argenziano M, Sparti R, Bessone F, Bianco G, Bastiancich C, Castagnoli C, Stella M, Musso T, Cavalli R. (2021) Enhanced Antimicrobial and Antibiofilm Effect of New Colistin-Loaded Human Albumin Nanoparticles. Antibiotics (Basel, Switzerland) 10(1) [DOI PMID]
Argenziano M, Gigliotti CL, Clemente N, Boggio E, Ferrara B, Trotta F, Pizzimenti S, Barrera G, Boldorini R, Bessone F, Dianzani U, Cavalli R, Dianzani C. (2020) Improvement in the Anti-Tumor Efficacy of Doxorubicin Nanosponges in In Vitro and in Mice Bearing Breast Tumor Models. Cancers 12(1) [DOI PMID]
Clemente N, Boggio E, Gigliotti LC, Raineri D, Ferrara B, Miglio G, Argenziano M, Chiocchetti A, Cappellano G, Trotta F, Caldera F, Capucchio MT, Yagi J, Rojo MJ, Reno F, Cavalli R, Dianzani C, Dianzani U. (2020) Immunotherapy of experimental melanoma with ICOS-Fc loaded in biocompatible and biodegradable nanoparticles. Journal of controlled release : official journal of the Controlled Release Society 320 112-124 [DOI PMID]
Donalisio M, Argenziano M, Ritta M, Bastiancich C, Civra A, Lembo D, Cavalli R. (2020) Acyclovir-loaded sulfobutyl ether-beta-cyclodextrin decorated chitosan nanodroplets for the local treatment of HSV-2 infections. International journal of pharmaceutics 587 119676 [DOI PMID]
Dianzani C, Monge C, Miglio G, Serpe L, Martina K, Cangemi L, Ferraris C, Mioletti S, Osella S, Gigliotti CL, Boggio E, Clemente N, Dianzani U, Battaglia L. (2020) Nanoemulsions as Delivery Systems for Poly-Chemotherapy Aiming at Melanoma Treatment. Cancers 12(5) [DOI PMID]
Fathy Abd-Ellatef GE, Gazzano E, Chirio D, Hamed AR, Belisario DC, Zuddas C, Peira E, Rolando B, Kopecka J, Assem Said Marie M, Sapino S, Ramadan Fahmy S, Gallarate M, Abdel-Hamid AZ, Riganti C. (2020) Curcumin-Loaded Solid Lipid Nanoparticles Bypass P-Glycoprotein Mediated Doxorubicin Resistance in Triple Negative Breast Cancer Cells. Pharmaceutics 12(2) [DOI PMID]
Chirio D, Peira E, Dianzani C, Muntoni E, Gigliotti CL, Ferrara B, Sapino S, Chindamo G, Gallarate M. (2019) Development of Solid Lipid Nanoparticles by Cold Dilution of Microemulsions: Curcumin Loading, Preliminary In Vitro Studies, and Biodistribution. Nanomaterials (Basel, Switzerland) 9(2) [DOI PMID]
Conniot J, Scomparin A, Peres C, Yeini E, Pozzi S, Matos AI, Kleiner R, Moura LIF, Zupancic E, Viana AS, Doron H, Gois PMP, Erez N, Jung S, Satchi-Fainaro R, Florindo HF. (2019) Immunization with mannosylated nanovaccines and inhibition of the immune-suppressing microenvironment sensitizes melanoma to immune checkpoint modulators. Nature nanotechnology 14(9) 891-901 [DOI PMID]
Gnaim S, Scomparin A, Eldar-Boock A, Bauer CR, Satchi-Fainaro R, Shabat D. (2019) Light emission enhancement by supramolecular complexation of chemiluminescence probes designed for bioimaging. Chemical science 10(10) 2945-2955 [DOI PMID]
Jadhav SA, Brunella V, Sapino S, Caprarelli B, Riedo C, Chirio D, Gallarate M. (2019) Poly (N-isopropylacrylamide) based hydrogels as novel precipitation and stabilization media for solid lipid nanoparticles (SLNs). Journal of colloid and interface science 541 454-460 [DOI PMID]
Malfanti A, Scomparin A, Pozzi S, Gibori H, Krivitsky A, Blau R, Satchi-Fainaro R, Mastrotto F, Caliceti P, Salmaso S. (2019) Oligo-guanidyl targeted bioconjugates forming rod shaped polyplexes as a new nanoplatform for oligonucleotide delivery. Journal of controlled release : official journal of the Controlled Release Society 310 58-73 [DOI PMID]
Muntoni E, Marini E, Ahmadi N, Milla P, Ghe C, Bargoni A, Capucchio MT, Biasibetti E, Battaglia L. (2019) Lipid nanoparticles as vehicles for oral delivery of insulin and insulin analogs: preliminary ex vivo and in vivo studies. Acta diabetologica 56(12) 1283-1292 [DOI PMID]
Muntoni E, Martina K, Marini E, Giorgis M, Lazzarato L, Salaroglio IC, Riganti C, Lanotte M, Battaglia L. (2019) Methotrexate-Loaded Solid Lipid Nanoparticles: Protein Functionalization to Improve Brain Biodistribution. Pharmaceutics 11(2) [DOI PMID]
Nunes F, Rodrigues M, Ribeiro MP, Ugazio E, Cavalli R, Abollino O, Coutinho P, Araujo ARTS. (2019) Incorporation of Cro thermal water in a dermocosmetic formulation: cytotoxicity effects, characterization and stability studies and efficacy evaluation. International Journal of Cosmetic Science 41 (6), 604-612 [DOI PMID]
Sapino S, Peira E, Chirio D, Chindamo G, Guglielmo S, Oliaro-Bosso S, Barbero R, Vercelli C, Re G, Brunella V, Riedo C, Fea AM, Gallarate M. (2019) Thermosensitive Nanocomposite Hydrogels for Intravitreal Delivery of Cefuroxime. Nanomaterials (Basel, Switzerland) 9(10) [DOI PMID]
Caldera F, Argenziano M, Trotta F, Dianzani C, Gigliotti L, Tannous M, Pastero L, Aquilano D, Nishimoto T, Higashiyama T, Cavalli R. (2018) Cyclic nigerosyl-1,6-nigerose-based nanosponges: An innovative pH and time-controlled nanocarrier for improving cancer treatment. Carbohydrate polymers 194 111-121 [DOI PMID]
Chirio D, Peira E, Sapino S, Dianzani C, Barge A, Muntoni E, Morel S, Gallarate M. (2018) Stearoyl-Chitosan Coated Nanoparticles Obtained by Microemulsion Cold Dilution Technique. International journal of molecular sciences 19(12) [DOI PMID]






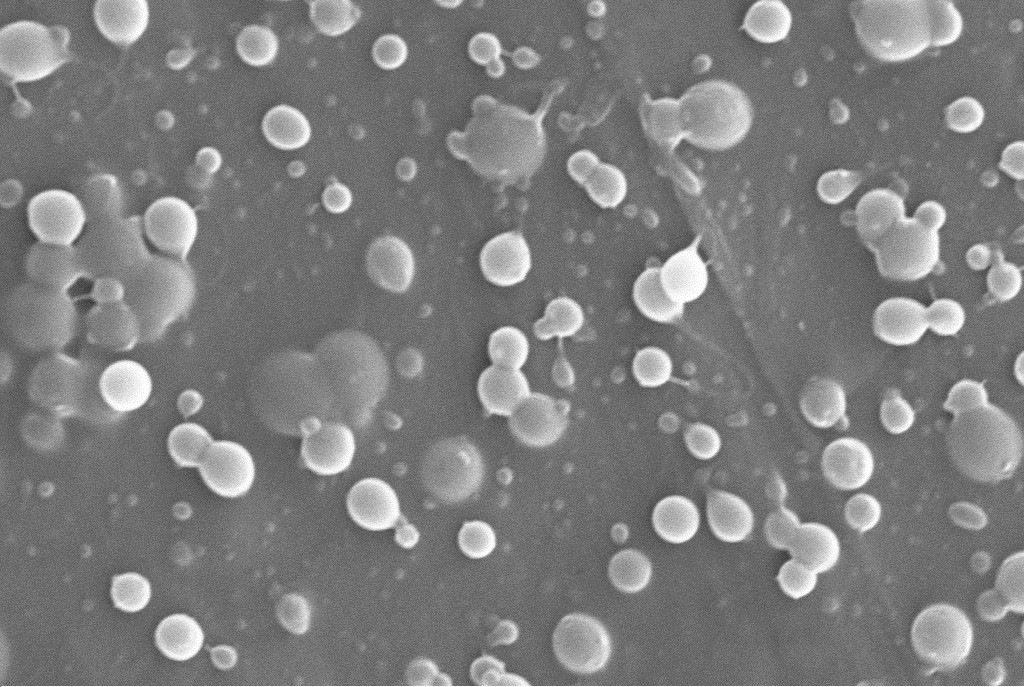 Solid lipid nanoparticles
Solid lipid nanoparticles In-situ gelling microemulsions
In-situ gelling microemulsions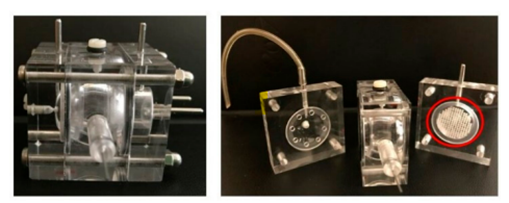 Ocular 3-compartments flow cell
Ocular 3-compartments flow cell
 simulatori della luce solare
simulatori della luce solare